Molecular sieves SLOVSIT
 Composition and structure
Composition and structure
High-quality synthetic zeolites have composition and properties similar to chemical substances. That is why the properties of the molecular sieves SLOVSIT correspond to the general characteristics of synthetic zeolites.
Zeolite molecular sieves, both natural and synthetic, are crystalline metal-alumino-silicates. They have a 3-D-network structures. Basic building units are tetrahedrons [SiO4]-4 a [AlO4]-5 in which the silicon and aluminum atom deposited centrally and the oxygen atoms are located in the corners of tetrahedrons. The tetrahedrons are linked together with oxygen atoms common to two adjacent tetrahedrons. The negative grid charge is neutralized by positive metallic cations. In both zeolites, they are, as a rule, cations univalent or bivalent metals or theirs combinations.
The chemical composition of zeolites is expressed the general formula in the anhydrous form:
Me2/m.Al2O3 .nSiO2
where Me is metal ion, mostly alkali metal Na+, K+ or metal of alkaline earth, most often Ca+2, or Mg+2 or Ba+2,
m is valence of the respective metal and n is the moles ratio SiO2:Al2O3, or n/2 = Si:Al that is also called module and a significant part of the zeolite.
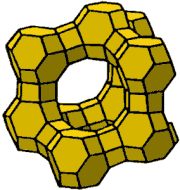
The basic structural unit of type zeolite A, X, Y are truncated cubo-octahedrons being also known as soda-lite cells. They are created from 24 elementary tetrahedrons [SiO4][AlO4]. The mutual ratio of tetrahedrons, i.e. module and the crystallographic structure are characteristic for the type of zeolite.
At the surface of the cubo-octahedron 6 four-membered oxygen rings in the octahedron symmetry and 8 six-membered oxygen rings in the tetrahedron symmetry are found. The mutual interconnection of cubo-octahedrons are interlinked together by means of bridges built up from oxygen atoms. The mode of connection determines the crystallographic structure and is characterized for zeolites type A, or X and Y.
Each cobo-octahedron in the A type zeolite is bound with six further cobo-octahedrons by means of four-membered oxygen bridges. Elementary cell of the A zeolite contains a combination of 24 silicon ions and aluminum the ratio of which corresponds to the characteristic module of the zeolite, 0,9-1 and 48 oxygen ions. Eight cubo-octahedrons linked through four-membered oxygen bridges create so called big cavities with a diameter 11,4.10-10m and volume 0.28 cm3/g, which is accessible through six holes, i.e. pores. Diameters of those inlet pores in the type A zeolite are limited by 8-membered oxygen ring and cations localized in its neighborhood.
The crystallographic structure of X and Y zeolites is identical with the natural faujasite. Hence, those types of zeolites are called faujasites. Their aluminosilicate skeleton is built up in a similar way as for zeolite A from the cubo-octahedron units, but linked with oxygen bridges created from 6-membered oxygen rings. Each cubo-octahedron is connected with four further cubo-octahedrons in the tetrahedronic arrangement. The elementary cell of zeolite type X and Y is built up from eight cubo-octahedrons and sixteen oxygen bridges. The adsorption space of the big cavities is surrounded by 10 cubo-octahedrons at faujasites. These cavities are accessible through four holes with a diameter 7,4.10-10 m is delimited by 12-membered oxygen ring, characteristic for type X and Y zeolites.
Modifications
Synthetic zeolites are produced as primary product in a sodium form as a rule. The modifications mean zeolites derived from the primary types by cations exchange, e.g. introduction of other metallic ions into zeolite structure. The ion exchange is carried out by contacting the zeolite with a salt solution of the respective metal or salts combinations in most cases. This exchange can’t be done up to 100%. That is why, even if the exchange of one metal only for the genuine is concerned, the resulting zeolite contains the combination of both. It results from the principle of electrical neutrality of the elementary cell that the higher is the module Si:Al, the lower quantity of cations neutralizing the negative charge of the grid is lower.
Type A zeolites are marketed predominantly as modifications with potassium, calcium cations and, in the basic modification with sodium cations, type X zeolites in sodium and calcium form, and zeolite Y in sodium form. Besides them, zeolites are also manufactured with other metals and diverse metal combinations or decationized (the metal is replaced by hydrogen cation) for special adsorption-separation and catalytic processes.
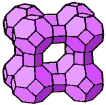
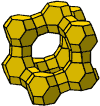
Type A zeolite Type X and Y zeolite
Effective pores radius
In the proximity of 8 or 10-membered oxygen rings delimiting the diameter of inlet holes into big cavities of basic zeolites, there is the localized part of cations. Cations with a bigger ion radius decrease the passage capacity through the pores. Introduction of cations with a higher valency or the increase of module Si:Al in the zeolite means the proportional decrease of cations quantity and thus, the increase of pores passage capacity. Pores in a certain zeolite type may be influenced by various factors, and that is why their passage capacity is characterized by the effective pores diameter.
The kind of exchanged or prevailing cations has thus a significant influence on the effective pores diameter for basic zeolite, but for the A type zeolites, in particular. Potassium whose ion radius (1,33.10-10 m) is the biggest cations, K+, Na+, and Ca+2 creates the zeolite modification KA or, more properly, KnaA with the smallest effective pores radius 3.10-10 m, and therefore, this zeolite is also labeled as 3A. Zeolit CaA, or CaNaA or 5A represent the modification of the type A with the biggest effective pores diameter 5.10-10 m. Calcium has admittedly ion radius (1,05.10-10m) bigger by 0,1.10-10m than sodium (0,95.10-10m), but in this case, the half count of calcium ions that exchange the sodium ions is decisive for the size of the effective ion diameter. The 3A, 4A, 5A type of zeolites represent the adsorbents with an ideal spacing between the unified dimensions of inlet channels into adsorption cavities from the point of view of separation of diverse molecules and production of important compounds.
Zeolites of X and Y type have in their sodium and calcium modification the effective pore radius within the range 8 – 12.10-10 m. In terms of the molecule radius, they are wide-pore zeolites where mostly not the sieve effect is used but a high adsorption capacity and selectivity towards polar and polarizable molecules.
Critical molecule diameter
The critical molecule diameters are characteristic dimensions in terms of adsorption allowing the assessment of passage capacity of adsorbates through the effective pore diameter of the respective type and modification of the molecular sieve. For the adsorbate molecules having their critical diameter smaller or comparable to the effective pore diameter, the inner adsorption surface of the zeolite is free accessible. The zeolites A, X, Y having theirs inlet pores of a circular cross-section is sufficient to describe molecules by one value of the critical diameter.
Properties
Such an adsorbent to be used in an industrial process must dispose of a sufficient capacity, selectivity, kinetic properties, strength, abrasion resistance, bulk density and thermal stability. These
properties determine the volume of packing, adsorbent dimensions for the required performance, shelf life and product quality from the given process. There is an presumption for synthetic zeolites that the dimension selectivity which is the basis for the sieve effect, will be fulfilled while observing the correct and verified technology, being analytically controlled at the decisive production stages.
The zeolite adsorbents are prepared for the practical application by forming adapted to particles of various shapes and size. Before forming, e.g. by pelletisation into balls or extrusion to the roller extrudate, the powdered zeolite is mixed with the binder. As binders for the zeolite granulation the natural aluminosilicates, such as kaolin clay, bentonite, halloysite, are mostly used. The binder conditions and facilitates forming particles and, at the same time, has an influence positively and negatively the mentioned properties of the granulated synthetic zeolites.
Equilibrium adsorption capacity (EAC)
It is one of the basic physical properties of the adsorbent that might be expressed for one particular condition (pressure, relative humidity and temperature. The complex characteristic EAC is the adsorption isotherm or various adsorption isotherms for the given adsorbent and the adsorbate at a certain adsorbate pressure intervals. The EAC of the high-quality powdered zeolites, without binder, is for identical types and modifications comparable with minimum deviations. The EAC is under influence of a binder quantity at granulated zeolites, where the quantity is practically inert in terms of the adsorption capacity. As a rule, the EAC of the granulates is by 20 % lower than that of pure powdered zeolites, which corresponds to the binder addition.
The EAC of synthetic zeolites SLOVSIT is determined for water at the temperature 25 °C and relative humidity 43%. Besides this, the complex course of adsorption isotherms for water and some selected adsorbates was determined.
Selectivity
Selectivity of zeolite with regard to adsorbates is manifested in several forms. Dimensional selectivity is a consequence of the diameter uniformity of effective pores, characterized for individual types and modifications of zeolites. Sieves effect is based on the dimensional selectivity and is so accurate to allow the quantitative division of some molecules according to their critical diameter. The most significant separation process based on the principle of dimensional selectivity is the industrial production of straight-chain hydrocarbons, n-alkanes using the adsorptive separation at the 5A type molecular sieves.
Another selectivity form is the different affinity of a zeolite towards molecules that penetrated to the adsorptive surface through the sieve of the dimensional selectivity. Heterogeneous surface of zeolites and the electrostatic fields induced by the metal cations prefer the adsorption of polar and polarizable molecules to non-polar and prefer molecules with unsaturated bonds to molecules with saturated bonds. Such a form of selectivity is implemented at the removal of polar impurities from gaseous and liquid technological streams but the most important industrial large scale process is the isolation of para-xylene from the mixture of C8 aromatic hydrocarbons on synthetic faujasites, that means on zeolites X and Y, with a special combination of exchanged cations.
Kinetic selectivity is a manifestation of differing speed of diffusion of molecules through pores of molecular sieves. Molecules with a smaller critical diameter diffuse faster than the bigger molecules, which generates a kinetic separation effect that can be practically used, e. g. at separation or final purification of air components.
Strength and abrasion stability of the granulated zeolites must reach such a level to preserve the long-term integrity of the particles and their shape under their application conditions. Strength in SLOVSIT zeolites is expressed as a force whose action leads to particles destruction of the molecular sieve. An abrasion stability rate is the time at that the total destruction of the granules. Both mechanical characteristics are determined at the molecular sieves SLOVSIT under standardized conditions.
Bulk density expresses the mass of a volume unit of anhydrous powdered or granulated zeolite. It is again an important characteristic because it has an influence on the absorber dimensions with regard to the designed output. Bulk density of zeolites SLOVSIT is determine as the ratio of the mass of the freshly dried zeolite to its bulk volume using the standardized method.
Thermal stability is a characteristic property of zeolites. Calcination of the finished granulates is conducted at the last production stage of zeolites at temperatures 500 to 650 °C but at a low water vapor environment. Resistance to the influence of water vapor and temperature is called the hydrothermal stability. The higher is the module, the higher is its hydrothermal stability. For this reason, the type Y zeolite has the highest thermal but also hydrothermal stability.
Desorption – recovery
The majority of the adsorption process work with the cyclic system and a fixed bed of the absorbent, which means, the adsorption and recovery stage are alternating. Regeneration phase is technically more demanding and, as a rule, has the decisive influence on the separation efficiency. Regeneration principle consists in setting up such technological parameters of the process to give priority to the desorption over the adsorption from the thermodynamic point of view. It is obvious from the course of the isotherms that the adsorption capacity of zeolites decreases by lowering the partial pressure or the adsorbate concentration and by increasing the temperature. These are the crucial parameters and, at the same time their direction, preferring the desorption phase.
In reality, several desorption procedures are combined in the majority. For instance, at the thermal desorption that is running at temperatures between 180-300 °C, the adsorbent bed is heated up with hot gas stream acting as the stripping medium by pushing the partial pressure of the adsorbed components downwards. For the POTASIT type zeolite, to exceed the temperature 300 °C at adsorption is not recommended. If the process is carried out in the liquid phase, the adsorbent is heated with a liquid stream that, like for the gas, decreases the adsorbate concentration or acts as the eluent, thus the displacement medium. At the reversible adsorption-desorption cycle, the affinity of the adsorbent to the desorbent must be lower or similar as the affinity to the adsorbent component to be displaced.
Oxidizing regeneration of the adsorbent may be integral part of the working cycle of the absorber but may also be applied as the way of independent reactivation of the adsorbent in situ or ex situ if, in such a way, the adsorbent´s properties are recovered and, thus, it is economically more favorable than the replacement of the packing with a new one.
Overview of uses for synthetic zeolites Slovsit
It is indicated for the individual types
Summary
The synthetic zeolites SLOVSIT have proven their quality at several industries and production processes. Their most-spread deployment is as drying agent in the manufacture of double glazing of windows and doors, in drying and final purification of refinery and petroleum products, at cooling equipment and statical drying of various hardware, devices and effervescents.
Table 1 – Critical diameter of some important molecules
| Molecules | Critical diameter [10-10 m] |
|---|---|
| Helium | 2,0 |
| Hydrogen, acetylene | 2,4 |
| Oxygen, CO and CO2 | 2,8 |
| Nitrogen | 3,0 |
| Water | 3,2 |
| Ammonia, hydrogen sulphide | 3,6 |
| Argon | 3,8 |
| Methane | 4,0 |
| Ethylene, ethylene oxid | 4,2 |
| Ethane, methanol, ethanol | 4,4 |
| Methylmercaptan | 4,5 |
| Propane, n-butane through n-C22H46 | 4,9 |
| Propylene | 5,0 |
| Ethyl mercaptan, 1-butene, trans-2-butene | 5,1 |
| 1,3-butadiene | 5,2 |
| Chlorodifluoromethane | 5,3 |
| i-butene through i-C22H46 | 5,6 |
| Dichloro-difluoromethane | 5,7 |
| Cyclohexane | 6,1 |
| Toluene, p-xylene | 6,7 |
| Benzene | 6,8 |
| Tetrachloromethane | 6,9 |
| Chloroform | 6,9 |
| m-xylene | 7,1 |
| o-xylene | 7,4 |
| Triethylamine | 8,4 |
Table 2 – Physico-chemical properties of SLOVSIT zeolites
| Zeolit - obchodný názov | |||||
|---|---|---|---|---|---|
| Characteristics | Potasit | Nalsit | Calsit | YSIT | YSIT/P |
| Type | 3A | 4A | 5A | 13Y | 13Y |
| Effective pores radius (10-10 m) | 3 | 4 | 5 | 9 | 9 |
| MUDULE SiO2:Al2O3 (mol/mol) |
1,9-2,1 | 1,9-2,1 | 1,9-2,1 | 4,5-5,5 | 4,5-5,5 |
| Prevailing cations | K+ | Na+ | Ca2+ | Na+ | Na+ |
| Exchange degree of the prevailing cation, (%) | 50-70 | 90-100 | 70-80 | 90-100 | 99-100 |
| Equilibrium adsorption capacity for water at 25 °C, (g/100 g ads.) pH2O 1170 Pa (RV 50%) |
17 | 19 | 18 | 23 | 23 |
YSIT/P – modified zeolite Y with enhanced capacity
RH – relative air humidity
pH2O partial pressure of water vapors over the adsorbent
Table 3 – Mechanical properties of synthetic zeolites SLOVSIT
| Particle shape | Dimensions (mm) | Particle strength min. | Abrasion stability min. | Bulk density | |
|---|---|---|---|---|---|
| average | length | (N) | (h) | (kg/m3) | |
| Balls | 0,8 - 1,0 | - | 5 | 20 | 760 - 780 |
| Balls | 1,4 - 1,8 | - | 10 | 25 | 735 - 850 |
| Balls | 2,0 - 2,5 | - | 12 | 50 | 710 - 820 |
| Balls | 2,0 - 3,0 | - | 18 | 50 | 710 - 820 |
| Rollers | 1,5 | 2 až 5 | 8 | - | 580 - 760 |
| Rollers | 3,0 | 5 až 20 | 20 | - | 600 - 760 |

